Galaxy Therapeutics Incorporated brings nature to the bedside. Inspired by the Jellyfish.

Results from those who reached the follow-up time window | ||||||
|---|---|---|---|---|---|---|
Core Lab Findings | Post procedure N=33 | 24hr Follow-up N=33 | 3-month Follow-up N=25/33 | 6-month Follow-up N=17/33 | 12-month Follow-up N=9/33 | |
Occlusion Raymond Roy (RR) Scale | ||||||
Complete occlusion (RR I) | 33.3% (11/33) | 33.3% (11/33) | 76% (19/25) | 70.6% (12/17) | 77.8% (7/9) | |
Residual Neck (R II) | 18.2% (6/33) | 15.2% (5/33) | 8.0% (2/25) | 11.8% (2/17) | 0.0% (0/9) | |
Residual Aneurysm (R III) | 51.5% (17/33) | 51.5% (17/33) | 16.0% (4/25) | 17.7% (3/17) | 22.2% (2/9) | |
Occlusion WEB-IT Scale | ||||||
A and B Occlusion | 33.3% (11/33) | 39.4% (13/33) | 80% (20/25) | 76.5% (13/17) | 78.8% (7/9) | |
A | 30.3% (10/33) | 33.3% (11/33) | 76% (19/25) | 52.9% (9/17) | 77.8% (7/9) | |
B | 3.0% (1/33) | 6.1% (2/33) | 4.0% (1/25) | 23.5% (4/17) | 0.0% (0/9) | |
C | 24.2% (8/33) | 21.2% (7/33) | 12.0% (3/25) | 11.8% (2/17) | 0.0% (0/9) | |
D | 42.4% (14/33) | 39.4% (13/33) | 8.0% (2/25) | 11.8% (2/17) | 22.2% (2/9) | |
Proportion of subjects with successful aneurysm occlusion at 12 months without parent artery stenosis or retreatment, as determined by independent imaging core lab:
Ruptured aneurysms may be included according to the following criteria:
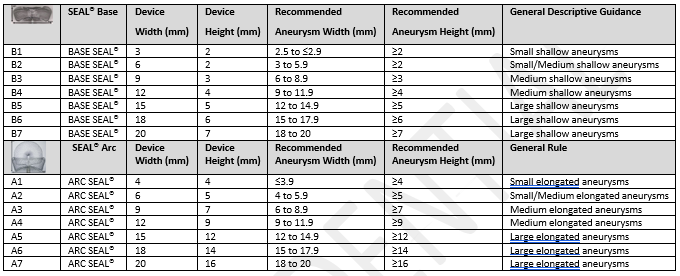
Serial | SEAL™ Base | Device | Device Width (mm) | Recommended Height (mm) Aneurysm Width (mm) | Recommended Aneurysm Height (mm) | General Descriptive Guidance |
|---|---|---|---|---|---|---|
B1 | BASE SEAL™ | 3 | 2 | 2.5 to $2.9 | 22 | Small shallow aneurysms |
B2 | BASE SEAL™ | 6 | 2 | 3
to 5.9 | 22 | Small/Medium shallow aneurysms Medium shallow aneurysms |
B3 | BASE SEAL™ | 9 | 3 | 6 to 8.9 | 23 | Medium shallow aneurysms |
B4 | BASE SEAL™ | 12 | 4 | 9 to 11.9 | 24 | Medium shallow aneurysms |
B5 | BASE SEAL™ | 15 | 5 | 12 to 14.9 | 25 | Large shallow aneurysms |
B6 | BASE SEAL™ | 18 | 6 | 15 to 17.9 | 26 | Large shallow aneurysms |
B7 | BASE SEAL™ | 20 | 7 | 18 to 20 | 27 | Large shallow aneurysms |
Serial | SEAL™ Base | Device | Device Width (mm) | Recommended Height (mm) Aneurysm Width (mm) | Recommended Aneurysm Height (mm) | General Descriptive Guidance |
|---|---|---|---|---|---|---|
A1 | ARC SEAL™ | 4 | 4 | $3.9 | 24 | Small elongated aneurysms |
A2 | ARC SEAL™ | 6 | 5 | 4 to 5.9 | 25 | Small/Medium elongated aneurysms |
A3 | ARC SEAL™ | 9 | 7 | 6 to 8.9 | 27 | Medium elongated aneurysms |
A4 | ARC SEAL™ | 12 | 9 | 9 to 11.9 | 29 | Medium elongated aneurysms |
A5 | ARC SEAL™ | 15 | 12 | 12 to 14.9 | 212 | Large elongated aneurysms |
A6 | ARC SEAL™ | 18 | 14 | 15 to 17.9 | 214 | Large elongated aneurysms |
A7 | ARC SEAL™ | 20 | 16 | 18 to 20 | 216 | Large elongated aneurysms |
